Epigenetic Inheritance
Transgenerational Epigenetic Inheritance: For most of the last century it was widely believed that, unlike genetic information, epigenetic information did not cross generational boundaries. In other words, epigenetic information was erased each and every generation such that offspring began life with a blank epigenetic slate. It is now known that this is not always the case as many examples of transgenerational epigenetic inheritance (TEI) have now been documented. Some of these examples include paramutation in plants, RNAi inheritance in C. elegans, and the inheritance of acquired traits in mammals. Non-coding RNAs and, in particular, small non-coding RNAs are major informational vectors for epigenetic inheritance in plants and animals. We are using exploring mechanisms by which non-coding RNAs direct epigenetic inheritance in animals.
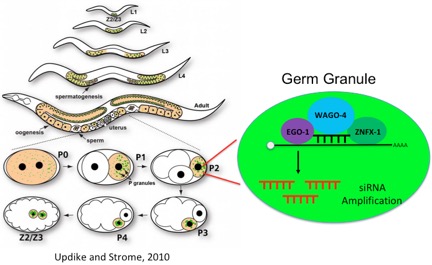
A class of non-coding RNA termed small interfering (si)RNA is associated with epigenetic inheritance in the metazoan model organism C. elegans. The Kennedy lab has conducted several genetic screens to identify cellular factors the allow siRNAs to drive epigenetic inheritance. These screens have identified two major pathways responsible for RNA directed epigenetic inheritance. One of these pathways is a nuclear pathway that allows siRNA to modify chromatin of genes undergoing epigenetic inheritance. More information on this pathway can be found via the non-coding RNA and Gene Regulation link. The second pathaway invovles cytoplasmic RNA binding and RNA modifying enzymes that inhabit germ granules, a type of phase separated organelle forming in germ cells. More information on germ granules and inheritance promoting factors localizing to germ granules can be found via the germ granules and phase seperation link, the transposons and pUG RNA link, and the genetic lags and hangovers link. The illustration below represents our current thinking on how nuclear and cytoplasmic pathways work together to promote RNA-directed epignetic inheritance in the germline. Interestingly, both the cytoplasmic and nuclear pathways are necessary for germline immortality, suggesting that siRNA-based epigenetic inheritance systems transmit important epigenetic signals across generations during the normal course of reproduction. We speculate that one major function of the RNA-directed epigenetic inheritance pathways is to allow parents to inoculate their progeny against expression of unwanted or parasitic genetic elements that inhabit the genomes of all organisms.
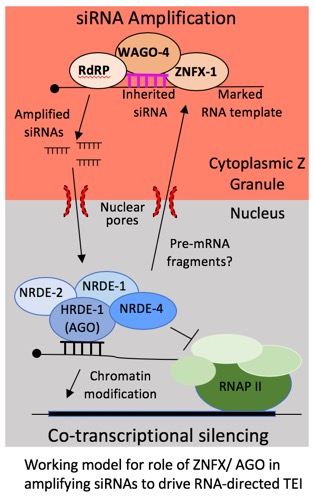
Potential Projects: Our recent studies identified two RNA binding proteins, ZNFX-1 and WAGO-4, as important mediators of RNA-directed epignetic inheritance (henceforth, transgenerational epigenetic inheritance or TEI). Both proteins localize to a liquid-like condensate that we name the Z granule (see link to germ granules and phase separation for more details). Because the Z granule segregates with the germline during development, we speculate that one function of the Z granule is to concentrate and segregate RNAs and proteins into the germline in order to promote RNA-based TEI. Interestingly, ZNFX-1 is a conserved RNA helicase and the closest homologue of ZNFX-1 is SMG-2/UPF1, which is an RNA helicase that marks mRNAs that contain premature termination codons for silencing via nonsense mediated decay. We are exploring the idea that ZNFX-1 functions analogously during TEI to physically mark mRNAs encoded by genes in need of heritable regulation. We are using genetic screens to identify cellular factors that allow the Z granule to form and segregate with the germline. Finally, we would like to revisit our genetic screen that identified ZNFX-1 and WAGO-4 (many genes are not yet cloned) to identify additional genes and pathways acting with ZNFX-1 and WAGO-4 to promote RNA-based epigenetic inheritance. Finally, we are interested in understanding why the C. elegans epigenetic inheritance systems are required for germline immortality. We would like to identify the epigenetic signals that pass across generations during the normal course of growth and reproduction and then assess how these epigenetic signals contribute to the immortality of the germline in animals. The image below is a working model for how germ granuels (also Z granules) might conentrate epigenetic inheritance driving RNAs and proteins within germ cells to promote multi-generational epigenetic inheritance. The image on the left was taken from the indicated source.
Non-coding RNA, Chromatin, and Gene Regulation
Nuclear RNAi: Epigenetics is the study of changes in gene expression or phenotypes that are not the result of changes in DNA sequence. Epigenetic pathways play important roles in gene regulation during reproduction, development, growth, and aging. Non-coding RNAs have emerged as important vectors for directing and regulating epigenetic processes in eukaryotes. For instance, long non- coding RNAs direct X-chromosome inactivation and imprinting in mammals, while small non-coding RNAs direct heterochromatin formation in yeast, co-transcriptional gene silencing in C. elegans, and genome rearrangement in Paramecium. The mis-regulation of epigenetic pathways contributes to the etiology of dozens of human diseases, including cancer. We are exploring how non-coding RNAs control and regulate gene expression programs and epigenetic states in animals. We expect the work we are doing to increase our understanding of how non-coding RNA reprograms epigenetic states and, therefore, may help us understand and, possibly, treat diseases in people.
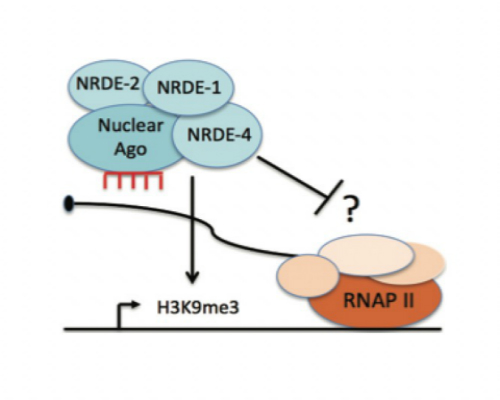
Small regulatory RNAs such as siRNAs, miRNAs, and piRNAs are short non-coding RNAs expressed in most eukaryotic cells. These small RNAs are bound by Argonaute (AGO) or PIWI proteins and act as specificity factors (guide RNAs) to regulate other homologous RNAs at the level of transcription, stability, and translation. In most eukaryotes, small RNAs act within nuclei where they regulate genome stability, heterochromatin formation, and transcription. For example, the data below show a GFP-tagged Ago that localizes to the nuclei of somatic cells only when bound to siRNAs which base pair with nascent RNAs. We have established C. elegans as a model system for studying how small RNAs regulate gene expression within animal nuclei. We have used genetic screens to identify components of the nuclear RNAi machinery (NRDE-1/2/3/4 and HRDE-1). According to our current model, AGO proteins such as HRDE-1 and NRDE-3 bind siRNAs in the cytoplasm and escort these guide RNAs into nuclei where they bind (via Watson-Crick base pairing) to nascent transcripts (pre-mRNAs). Once bound to pre-mRNAs, AGOs recruit the downstream nuclear RNAi effectors (NRDE-1/2/4), which assemble in a hierarchical manner on pre-mRNAs. The NRDEs then recruit enzymes that modify chromatin (e.g. H3K9me3) and inhibit RNA Polymerase II (RNAPII) during the elongation step of transcription. Recent studies in the lab have shown that the nuclear RNAi machinery is particularly important for the transmittal of small RNA-based epigenetic information across generations.
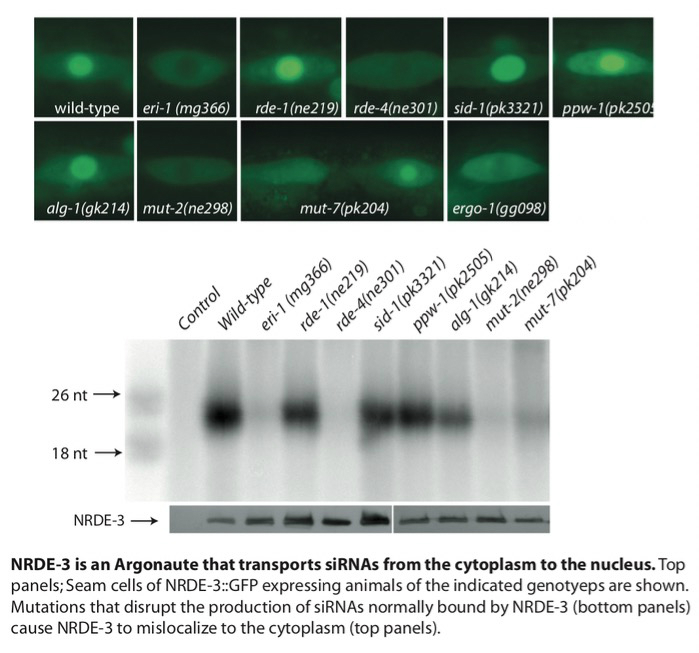
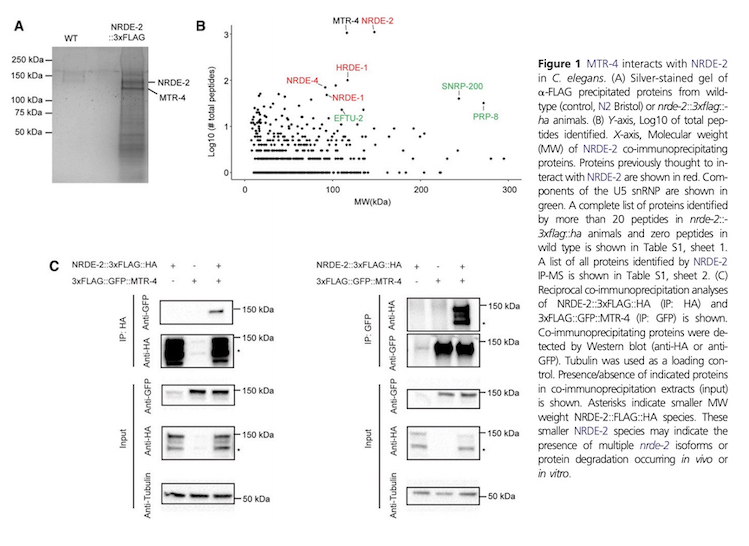
Potential Projects: We are currently investigating how the NRDEs regulate chromatin states and RNAPII transcription. We recently conducted proteomic and genetic analyses that identified additional components of the C. elegans nuclear RNAi machinery. One factor identified by these studies was the the conserved helicase MTR-4 that interacts physically with NRDE-2 in both C. elegans and mammalian cells and is a core component of the C. elegans nuclear RNAi machinery. The image below shows immunoprecipitation and mass spectrometry (IP-MS) data and co-IP analysis of both worm and human NRDE-2 and MTR-4. We would like to further explore how the NRDE-2/MTR-4 complex regulates gene expression in animals. We are testing the idea that NRDE-2/MTR-4 regulates gene expression via a novel mechanism we refer to as abortive splicing. The ancient and conserved nature of small RNA-directed co-transcriptional gene regulation and NRDE-2/MTR-4 interactions hints that studying nuclear RNAi in model organisms such as C. elegans could lead to insights into gene regulatory systems and epigenetic pathways that are applicable to all eukaryotes.
Germ Granules and Phase Separation
The Z granule: Liquid droplet organelles (also referred to as biomolecular condensates) are non-membrane-bound, dynamic concentrations of protein and RNA that coalesce spontaneously from the cytoplasm or the nucleoplasm via a process termed liquid-liquid phase separation. Like oil and water, liquid droplet organelles and their surrounding cytoplasm or nucleoplasm co-exist as separate states of liquid. Examples of condensates include nucleoli, processing (P) bodies, Cajal bodies, and germ granules. Current models posit that many condesnates exist to concentrate cellular reactants together in space and time in a way that facilitates biochemical reactions such as RNA processing and gene regulation.
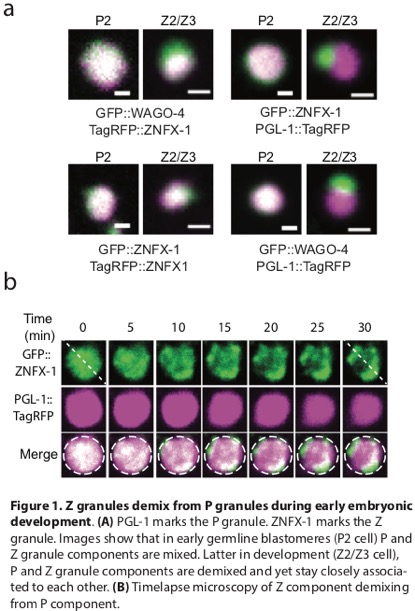
Recently, we identified two RNA-binding proteins, ZNFX-1 and WAGO-4 that bind and mark RNAs encoded by genes undergoing heritable gene silencing. Interestingly, both ZNFX-1 and WAGO-4 localize to a condensate that segregates with the germline during development. In early germline blastomeres, ZNFX-1 and WAGO-4 colocalize with components that define a distinct condensate termed the P granules; however, later in development, ZNFX-1 and WAGO-4 demix/separate from P granule factors to form a distinct condensate that we term the Z granule. The image below shows the demixing of ZNFX-1 from PGL-1 in the P3/P4 blastomeres. In adult germ cells, Z granules assemble into an ordered tri-droplet assemblage with two other condensates (P granules and Mutator foci, also M granules). We refer to this ordered three condensate assemblage, of which Z granules are the bridge, as the PZM holodroplet. Our work has shown that condensates can undergo developmentally regulated cycles of mixing/demixing and can assemble into spatially ordered multi-droplet structures. Given that ZNFX-1 and WAGO-4 are required for RNAi-based heritable gene regulation, we speculate that the spatiotemporal ordering of condensate formation coordinates the complex RNA processing and RNA regulatory steps underlying gene regulation and germ cell function.
Potential Projects: The image directly below shows an example of a PZM "holodroplet". Red indicates the Z granule. A long-term objective of the lab is to understand how condensates organize the cytoplasm and how this organization contributes to regulatory processes such as RNA-directed epigenetic inheritance and germ cell totipotency. We are conducting genetic screens to identify factors that direct the spatiotemporal ordering of the PZM, proteomics and RNA IP experiments on Z granule factors to identify RNA/protein constituents of the Z granule, and CRISPR-Cas9 based epitope tagging of RNAi factors to identify additional proteins that localize to Z granules. These latter studies have recently led us to identify at least two additional new condesates. Future studies will use genetic screens to identified genes and pathways required for PZM formation and maintenance, develop cell-biological approaches to explore how RNAs transit the PZM, and super resolution microscopy to investigate how components of the PZM mix and demix during germline development.

Regulators of Epigenetic Inheritance
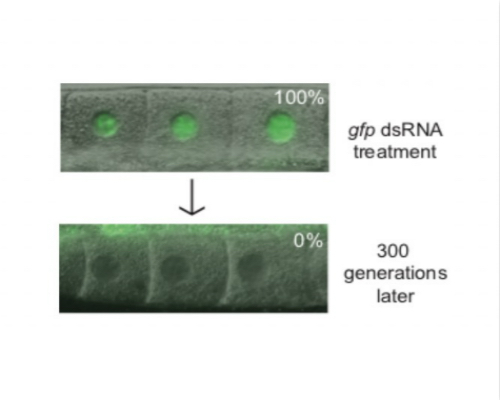
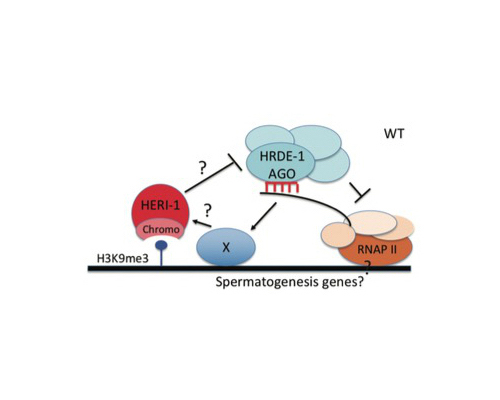
Brakes on Epigenetic Inheritance: Transgenerational epigenetic inheritance (TEI) is typically limited to a small number (1-3) of generations. In a few cases, such as paramutation in plants or RNAi inheritance in C. elegans, inheritance can be remarkably long-lasting (>10 generations). The molecular mechanism(s) setting the number of generations for which epigenetic information is inherited are not known. We wondered if C. elegans, and perhaps all animals, possess genetically encoded systems that limit the transgenerational perdurance of epigenetic inheritance. If so, we predicted we should be able to identify these systems by identifying mutations that cause inheritance to last longer than normal. Indeed, we conducted a forward genetic screen and identified 20 mutations that caused RNAi inheritance to last for >7 generations longer than in wild-type animals. In two cases, RNAi-based gene silencing becomes essentially permanent (>300 generations). One gene identified by our screen was a gene we term heritable enhancer of RNAi-1 (heri-1). heri-1 encodes a germline-expressed nuclear protein that contains a chromodomain and a kinase-like domain. HERI-1 is expressed in germ cells and is physically recruited to genes undergoing heritable silencing. The physical recruitment of HERI-1 to genes undergoing heritable silencing suggests that HERI-1 may have a direct and dedicated role in limiting TEI. Finally, heri-1(-) animals have spermatogenesis defects, which are suppressible by mutation in the small RNA-binding protein HRDE-1, suggesting that HERI-1 might normally act in sperm progenitor cells to limit small RNA-based inheritance in male germ cells.
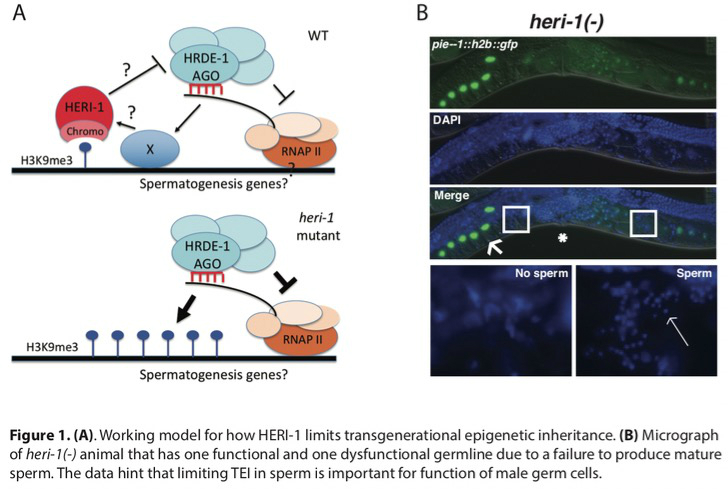
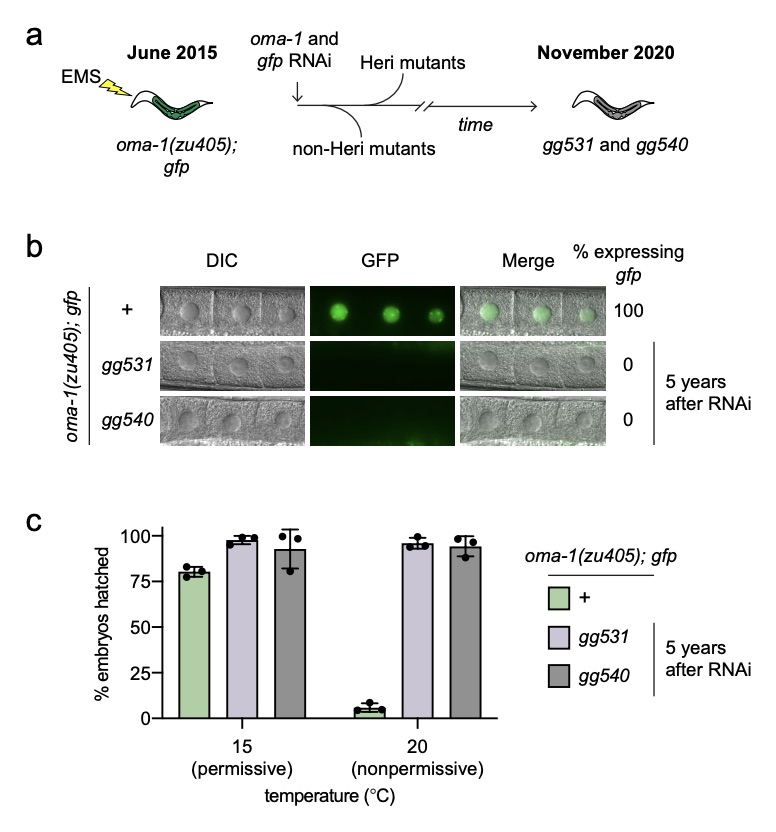
We revisited the genetic screen described above recently and identified another system used by C. elegans to limit TEI. Our recent work on pUG RNAs shows that during RNAi inheritance, gene silencing is transmitted by a self-perpetuating cascade of siRNA-directed poly(UG) tailing of mRNA fragments (pUGylation), followed by siRNA synthesis from poly(UG)-tailed mRNA templates (termed pUG RNA/siRNA cycling). Despite the self-perpetuating nature of pUG RNA/siRNA cycling, RNAi inheritance is finite. Our genetic screen led us to the realization that, in the absence of Piwi-interacting RNAs (piRNAs), an animal-specific class of small noncoding RNA, RNAi-based gene silencing can become essentially permanent, lasting at near 100% penetrance for more than five years and hundreds of generations. This permanent gene silencing is mediated by perpetual activation of the pUG RNA/siRNA TEI pathway. Further, we find that piRNAs coordinate endogenous RNAi pathways to prevent germline-expressed genes, which are not normally subjected to TEI, from entering a state of permanent and irreversible epigenetic silencing also mediated by perpetual activation of pUG RNA/siRNA cycling. Together, our results show that one function of C. elegans(-) piRNAs is to insulate germline-expressed genes from aberrant and runaway inactivation by the pUG RNA/siRNA epigenetic inheritance system. The data shown below is Figure 1 from an about to be published paper describing the role of piRNAs in limiting TEI.
Potential projects:The physical recruitment of HERI-1 to genes undergoing transgenerational epigenetic regulation suggests that C. elegans possess systems specifically dedicated to regulating TEI. Future studies in the lab will continue to explore this idea. We are very interested in understanding how HERI-1 is recruited to genes undergoing TEI and what HERI-1 does at these loci to limit TEI. We are also interested in understanding how nuclear small RNAs (via HRDE-1) and HERI-1 cooperate to promote spermatogenesis. Identifying the genes regulated by HERI-1 in sperm will be important to address this question. We have yet to clone many of the genes defined by the genetic screen that identified heri-1. We would like to continue identify these genes to further understand how HERI-1 limits TEI and, more generally, how C. elegans limits its potent epigenetic inheritance systems. Finally, we would like to know more about how piRNAs limit TEi, how the loss of piRNAs allows RNAi signals to become essentially permanent, and finally, ask if limiting TEI is a conserved function of animal piRNAs. Understanding how and why epigenetic signals are inherited and how they can become esentially "permanent" may impact our thinking on how C. elegans adapts to changing environmental conditions.
Genetic Lags and Hangovers
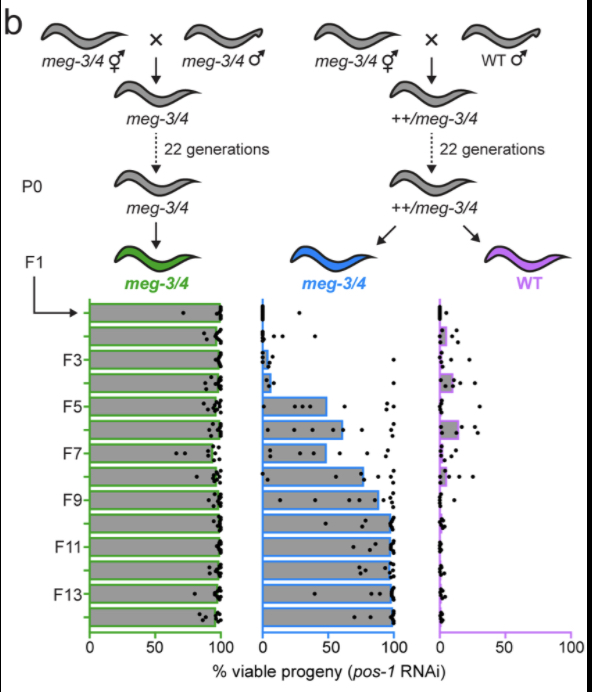
Non-Mendelian Inheritance: Genotype dictates phenotype. Recently, we identified several situations where genotype was transgenerationally disconnected from phenotype. For instance, germ granules are biomolecular condensates that form in the germ cells of all/most animals. We find that mutations that disrupt germ granule formation (such as mutations in the C. elegans meg-3/4 genes) cause defects in dsRNA-based gene silencing, however, this phenotype does not become apparent until five generations after animals are genetically mutant. We refer to this phenomenon as a phenotypic lag. The figure below shows an example of a phenotypic lag in which animals mutant for meg-3/4 do not become RNAi defective for many generations after introduction of the mutant allele. Amazingly, restoring these mutant animals to a wild-type genotype does not cause mutant phenotypes to revert to wild-type for an additional ten generations. We refer to this phenomenon as a phenotypic hangover. Given that C. elegans possesses systems to transmit epigenetic information across generations, it stands to reason that any genetic or environmental perturbations that alters the quality or quantity of germline epigenetic information could have heritable effects. Although such heritable alterations to the epigenome could conceivably be adaptive, it seems much more likely that such changes would have negative impacts on organismal fitness, which could, nonetheless, persist for generations. Such considerations may apply to other modes of TEI and other animals. It is important to note, however, that the lack of RdRP-like enzymes in many animals, suggests that if phenotypic hangovers or lags do occur in other animals, the generational perdurance of these phenotype/genotype disconnecsts will likely be limited compared to what we observe in C. elegans. Nonetheless, exploring how much phenotypic variation is contingent upon ancestral genotype in all animals, assessing the generational perdurance of this type of variation in different animals, and asking if phenotypic hangovers ever contribute to disease inheritance in humans will be important questions for future studies to address.
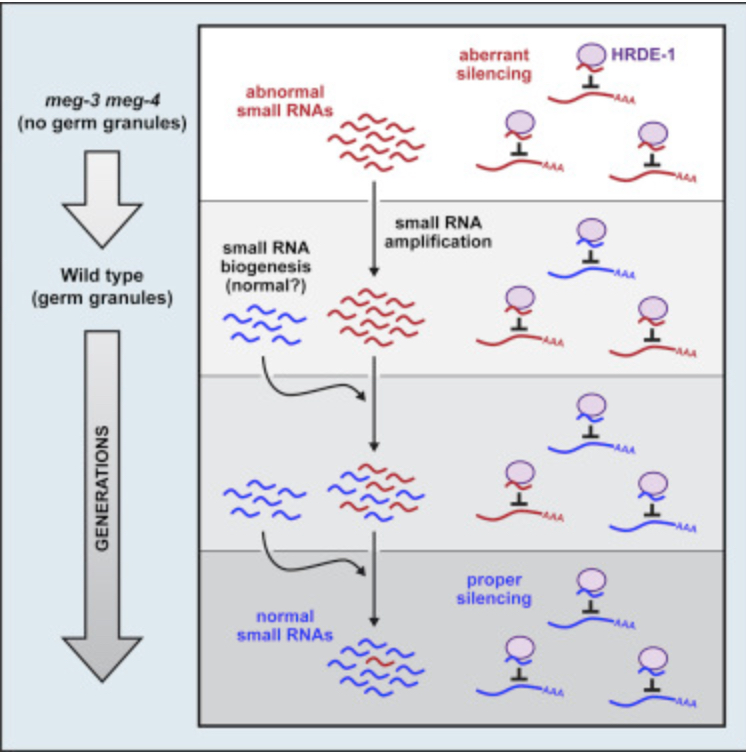
Potential Projects: We are contuing to explore the mechanism and generality of phenotypic lags and hangovers in animals. A working model showing our current thinking on how disrupted small RNA populations propagate themselves over generations is shown belo0w. We remain puzzled by the observation that the generational duration of lags and hangovers is unaffected by selection and would like to explore why this is so. We are also thinking of exploring a related idea that we call “structural Inheritance”. Proteins cause disease when they adopt pathological structures that convert newly translated proteins into the pathological state. Pathological protein structures are fairly common in nature and are linked to a number of proteinopathy diseases such as Alzheimer’s, ALS, and Parkinson’s disease. In yeast, recent studies suggest that protein structure can be inherited across mitotic and meiotic cell divisions. We are wondering if animal proteins ever assume structures or aggregations that become heritable when they form in the germline.
pUG-tailed RNAs:
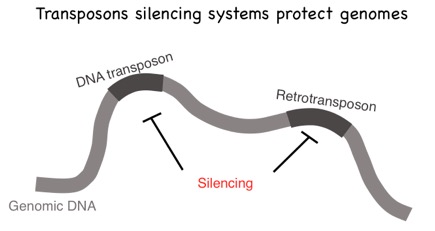
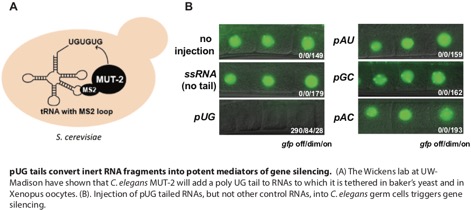
Transposons and Genome Defense: Nucleic acid parasites, such as transposons, and retrotransposons, live within the DNA of all living organisms. The first image below shows two common types of genome parasite termed DNA transposons and retrotransposons. These genomic parasites threaten genome integrity and cause disease when they reproduce, mobilize, and induce non-allelic recombination and/or disrupt genes. Mechanism(s) by which eukaryotic organisms protect themselves from nucleic acid parasites are not well understood. Nucleotidyl transferases (NTs) are polymerases that can add non-templated nucleotides to the 3’ termini of RNAs. For example, poly(A) polymerase adds long stretches of non-templated adenosines to the 3’ termini of mRNAs in eukaryotes. The C. elegans mut-2/rde-3 gene encodes a putative NT that was discovered in the 1980s as part of a genetic screen seeking genes required for transposon silencing. The Wickens lab at the University of Wisconsin Madison has shown that RDE-3/MUT-2 adds perfectly alternating repeats of uridine (U) and guanosine (G) nucleotides (termed poly UG or pUG tails) to the 3’ termini of RNAs to which it is tethered in yeast. We have now shown that, in its natural context in C. elegans, RDE-3 adds pUG tails to targets of RNAi, as well as to transposon RNAs. pUG tails with more than 16 perfectly alternating 3’ U and G nucleotides convert RNA fragments into agents of gene silencing. pUG tails promote gene silencing by recruiting RNA-dependent RNA polymerases (RdRPs), which use pUG-tailed RNAs (pUG RNAs) as templates to synthesize small interfering RNAs (siRNAs). Our results show that cycles of pUG RNA–templated siRNA synthesis and siRNA-directed mRNA pUGylation underlie dsRNA-directed transgenerational epigenetic inheritance in the C. elegans germline. We speculate that this pUG RNA/siRNA silencing loop allows parents to inoculate progeny against the expression of unwanted or parasitic genetic elements. The left panel of the second figure below illustrates the Wicken's lab system for studying NT enzymes and the right panel shows our data demonstrating that a UG tail turns an otherwise inert RNA into a potent mediators of gene silencing.
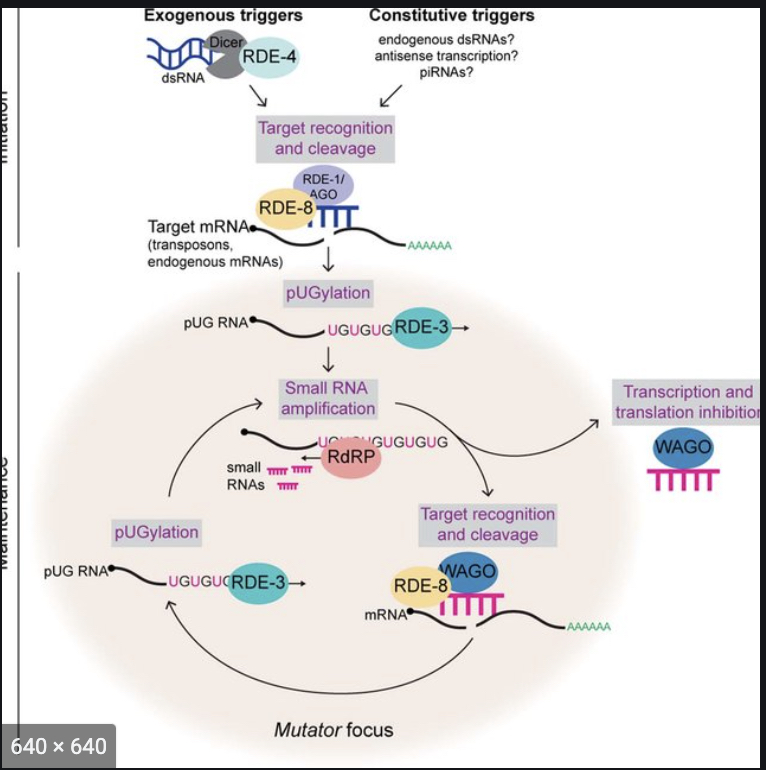
Potential Projects: We find that RDE-3 adds pUG tails to germline- and soma-expressed RNAs in C. elegans and we have also demonstrated a role for this modification in transposon silencing and TEI. We find that RdRPs are recruited, either directly or indirectly, to pUG tails and use pUG RNAs as templates for siRNA synthesis. Assemblage of RDE-3 and other proteins, like the endonuclease RDE-8 and the RdRP RRF-1, into germline condensates termed Mutator foci likely coordinates RNA target recognition, cleavage, pUGylation, and siRNA amplification (see model Figure below). How (and where in the cell) pUG tails are added to mRNA fragments and how RDE-3 "knows" which RNAs to pUGylate are interesting questions that we are interested in investigating further. We find that functional pUG tails consist of more than eight pairs of perfect or near-perfect 3’ UG repeats. These precise length and sequence requirements for pUG tail function hint that long pUG tails may impart stability upon mRNA fragments and/or form a structure which helps to recruit, and possibly prime, RdRPs, similar to the proposed role for poly(U)-tailing in small RNA–based gene silencing in Tetrahymena. Additionally, our data show that proteins other than RdRPs, such as TDP-1, the C. elegans ortholog of the mammalian UG-binding protein TDP-43, also interact with pUG repeats. We speculate that these other proteins may regulate the localization, stability, or function of pUG-tailed RNAs. We are collaborating with the Butcher and Wickens lab at UW-Madison to explore the idea that pUG tails may form a structure important for pUG function. Further, our results show that pUG RNAs act as informational vectors for TEI when they engage in feed-forward amplification cycles with RdRP-generated 2° siRNAs (See model below). These pUG/siRNA cycles, we speculate, allow C. elegans to remember past gene silencing events and inoculate progeny against expressing unwanted and/or dangerous genetic elements. Experimental RNAi-initiated pUG/siRNA cycles perdure for several generations, but are not permanent, suggesting that C. elegans possesses systems to prevent pUG/siRNA cycles from propagating in perpetuity. Interestingly, we find that RNAi-initiated pUG RNAs shorten progressively during TEI, suggesting that pUG RNA shortening, which may be an inevitable consequence of RdRP-based 2° siRNA synthesis, could function as one such brake on TEI. This is an idea worth testing. In contrast, the natural targets of pUGylation, such as transposons, are constitutively silenced by the pUG/siRNA system, suggesting that genetic systems, such as genomically encoded PIWI-interacting RNAs or endogenous dsRNAs, likely reinforce and refocus epigenetic pUG/siRNA silencing at these loci each generation. The logic of sense pUG RNA/antisense siRNA cycling resembles that of fly and mammalian piRNA “ping-pong” systems in which iterative base-pairing between genomically encoded sense/antisense transposon RNAs, and piRNAs derived from these RNAs, mediates stable transposon silencing. We speculate that related sense/antisense RNA systems may contribute to other biological processes where long-term memories of past expression states are needed, such as antiviral immunity, development, or inheritance of environmentally acquired traits. Finally, our work shows that long non-templated and non-homopolymeric tracts of ribonucleotides can be appended to, and confer novel functions to, RNAs in C. elegans. It will be of obvious interest to ask whether pUG-tailed RNAs, or RNAs bearing other unexpected tails, are restricted to C. elegans or are, instead, emissaries of a new class of eukaryotic RNA. Again, we look forward to testing this interesting idea.
Fissiparity in starfish: Many species of starfish have the remarkable ability to regenerate lost limbs. A few species of asteroideans (and brittle stars) divide asexually via a process termed fissiparity. During fissiparous reproduction, stars "rip" themselves in half and each half then regenerates into a complete starfish. A video of an Asterina star undergoing fissiparity can be seen here https://www.youtube.com/watch?v=wLOIi-cfgw4. Truly remarkable. Interestingly, a number of small (<1cm) species of Asterina and Brittle starfish are invasive to many home saltwater acquiria, indicating that these organims are easy to culture in controlled settings. We are wondering if the distinctive properties (small size, easy to grow, natural fissiparity) of these critters might make them good systems with which to study the molecular mechanismss of tissue regeneration. I am now growing several species of brittle and asterina stars in the lab now. The pictures below are taken from the web and resemble closely the stars I am currently growing. At present, I am trying to determine if any of my stars are capable of sexual reproduction. If yes, I will ask if I can manipulate germ cell DNA with CRISPR/Cas9. If yes...game on.